 |
The CCAMLR krill synoptic survey
|
Sampling Protocols
Net Sampling
Experience gained through participation in other international programmes
like BIOMASS has shown that standardization of equipment and methods is
one of the most crucial steps for any successful work during the field
sampling period and later analytical work. The following net sampling protocols
set out the procedures to be adopted so that participants carrying out
the CCAMLR Synoptic Survey 2000 can collect comparable high quality data
sets that will facilitate the establishment of a uniform and valuable database.
Objectives
There are two primary objectives for the net sampling programmme:
-
to validate and identify acoustic targets, confirming which targets can
be considered as krill and obtaining krill length frequency data for Target
Strength estimation
-
to describe krill demography and large scale distribution patterns of size
groups and maturity stages as well as regional recruitment indices.
These two objectives require two different sampling strategies which will
be outlined below
Standard Gear
The CCAMLR Working Group recommend the use of a standard type of net to
avoid potential variation in catchability and selectivity of nets. The
most appropriate type of net presently available is the RMT8+1
(Rectangular Midwater Trawl ; Baker et al. 1973). It was agreed at WG-EMM-99
that this net shall be used as the standard net for target and random hauls.
Alternative gear, such as an equivalent IKMT type of net of 8 to 10 m2
mouth opening, shall only be used if the RMT net is lost or damaged to
such a degree that it cannot be repaired. The net should preferably have
a mesh size of 3 to 4 mm. The net shall be equipped with a
flowmeter to estimate the filtered water volume as accurately as possible,
and a real-time time-depth-recorder (TDR) to follow the track of the net.
This page details the protocol for the RMT8 sampling. There are also
separate details for processing the zooplankton
from the RMT1.
Sampling strategy will be depend on whether a ship has an opening/closing
RMT or not.
-
Ships with an opening and closing RMT8 system will undertake a station
net haul at around local midnight, during the day these ships will
undertake a targetted net haul if suitable
targets are detected.
-
Ships without an opening and closing RMT8 system will undertake a station
net haul at around local midday and at around local midnight.
Station net hauls
Station net tows shall be carried out in the dark period of each day (around
local midnight). The timing of the midnight sample is constrained by the
period of darkness. A table of sampling times that take account of the
changes in length and time of the hours of darkness (due to variation in
date and position) will be produced for each ship. A midday station net
haul may also be carried out if target fishing has not taken place since
daybreak (see Target net haul protocol)
At each station a quantitative standard double oblique tow will be conducted
from the surface down to 200 m ( or to within 10 m of the bottom at stations
shallower than 200 m). Such a depth range is considered to be the best
compromise between the time available for sampling and the likely vertical
depth range of krill. During the hauls a constant ship's speed of 2.5 ±
0.5 knots is suggested. It is recommended to maintain a wire speed of 0.7
to 0.8 m/sec (42 to 48 m/min) during paying out and of 0.3 m/sec (18 m/min)
during hauling. The net mouth angle is remarkably constant during hauling
within the speed ranges given above. When the net reaches maximum depth,
the winch should be stopped for about 30 seconds to allow the net to stabilize
before starting to retrieve the net. If the net is hauled from the
stern of the ship then the propeller of the ship should be stopped when
the net reaches a depth of 15 to 20 m; this is to minimize the effects
of the propeller action on the net operation and avoids damage of the samples.
The total time of the net haul from surface to bottom to surface should
be 40 minutes.
The use of a real-time TDR is essential to maintain a smooth net trajectory
and control the maximum fishing depth. Calibrated flowmeters will be used
to give a measure of net speed during the haul as well as the total distance
travelled. The flowmeter should be mounted outside the net opening to avoid
clogging which may reduce the efficiency. The dependence of mouth
angle to the vertical of net speed has been investigated for the RMT system.
The formula of Pommeranz et al. (1982) should be be used to calculated
the filtered water volume for oblique hauls. (If horizontal hauls are used
then the formulas of Roe et al., 1980, should be used).
Target net hauls
Directed or targetted net sampling effort will be necessary to reduce the
uncertainty associated with the delineation of krill in the acoustic data
record (see planning meeting report para 10). This sampling would
be directed at a variety of “acoustic morphs”, some presumed to be krill
and some presumed not to be krill. During the daytime acoustic survey
period the following target fishing strategy should be adopted by those
ships that have an opening and closing RMT8 net system:
-
From the time of local apparent sunrise to local apparent noon conduct
a directed tow if an acoustic morph of interest is detected and a
reasonable chance of sampling it exists
-
If the directed tow is conducted between local apparent sunrise and
three hours before local apparent noon, delay the CTD cast until local
apparent noon
-
If a directed tow is conducted in the three hour period before local apparent
noon, conduct the CTD cast at the same locale
-
If no suitable acoustic morphs are detected by local apparent noon, conduct
a standard oblique tow in conjunction with a CTD cast at the midday station.
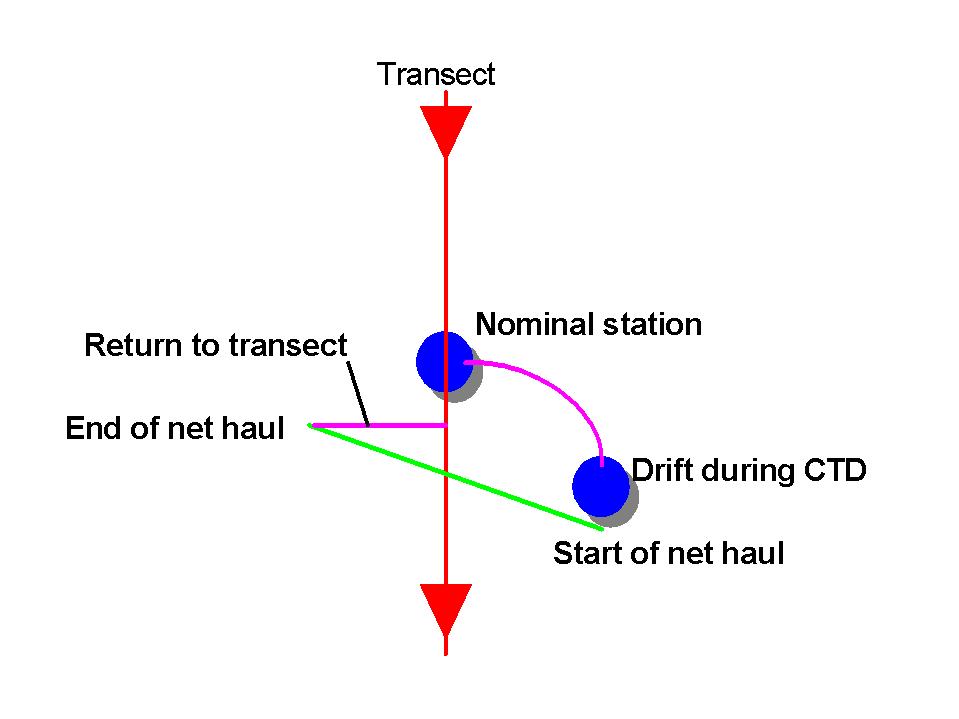
After the net haul the vessel will return directly by the shortest route
to the acoustic transect line and continue the acoustic transect.
Laboratory Sampling
Subsampling
Samples from RMT can range from a few grams to several kilograms in weight.
The total volume of the net catch should be measured (total drained sample
volume). For catches with a total volume of less than 1 litre all the sample
should be sorted. The minimum requirement is that all the krill and salp
specimens shall be counted and measured immediately after the catch. If
at all possible then the rest of the zooplankton either should be identified
to the species level and counted, or stored in 10% buffered formalin solution
for later analyses.
Samples that are too large to be sorted completely are subsampled volumetrically
immediately after the catch. Due to differences in catch composition, subsampling
must be carried out differently :
-
if the sample size is larger than 1 litre and the sample mainly consists
of krill : first the total drained sample volume has to be determined and
recorded, afterwards a 1 litre subsample is taken randomly from the total
samples and all krill and salp specimens are counted from this subsample
(for measurements see below). For the remaining zooplankton fraction see
above.
-
if the sample size is larger than 1 litre and the sample mainly consists
of salps : first the total drained sample volume has to be determined and
recorded, afterwards all krill have to be sorted from the total sample,
counted and measured (for measurements see below). Finally a 1 litre subsample
is taken randomly from the total samples and all salp specimens are counted
from this subsample. For the remaining zooplankton fraction see above.
Subsampling of catches should be undertaken on samples suspended in sufficient
seawater to ensure mixing. Total volume of sample and seawater should be
recorded, the sample should then be well mixed and a known volume of sample/seawater
mixture withdrawn rapidly. The subsample volume should be recorded and
the ratio of sample to subsample calculated and recorded.
Measurements of krill
Numeric
The reductions in sample size have to be recorded properly to allow the
extrapolation from the subsample to the total sample size for each of the
sample components (krill, salps, zooplankton). These data together with
information on fishing depth and filtered water volume will allow the necessary
standardization of krill densities and length density data (per m2
or per 1000 m3)
Volume and Weight
Total volume of krill in the sample (or subsample) should be measured.
Participants should use a displacement volume or a wet weight with an accuracy
of 0.1 grams. The method should be consistent throughout the cruise.
If time and resources permit data on length-weight relationships should
be established for different parts of the survey area (e.g. for each statistical
Subarea) and separately for 3 groups of krill (adult males, gravid females
and all other krill - see Morris et al., 1988 for further details of this
classification). If this is to be conducted on the ship then about 10 specimens
per length class should be pooled and weighted. The station number must
be recorded for each of the 10-specimen-measurement.
Length Measurements
The standard length measurement is total length as defined by the Discovery
method (AT) from the anterior margin of the eye to the tip of the telson
without the terminal spines 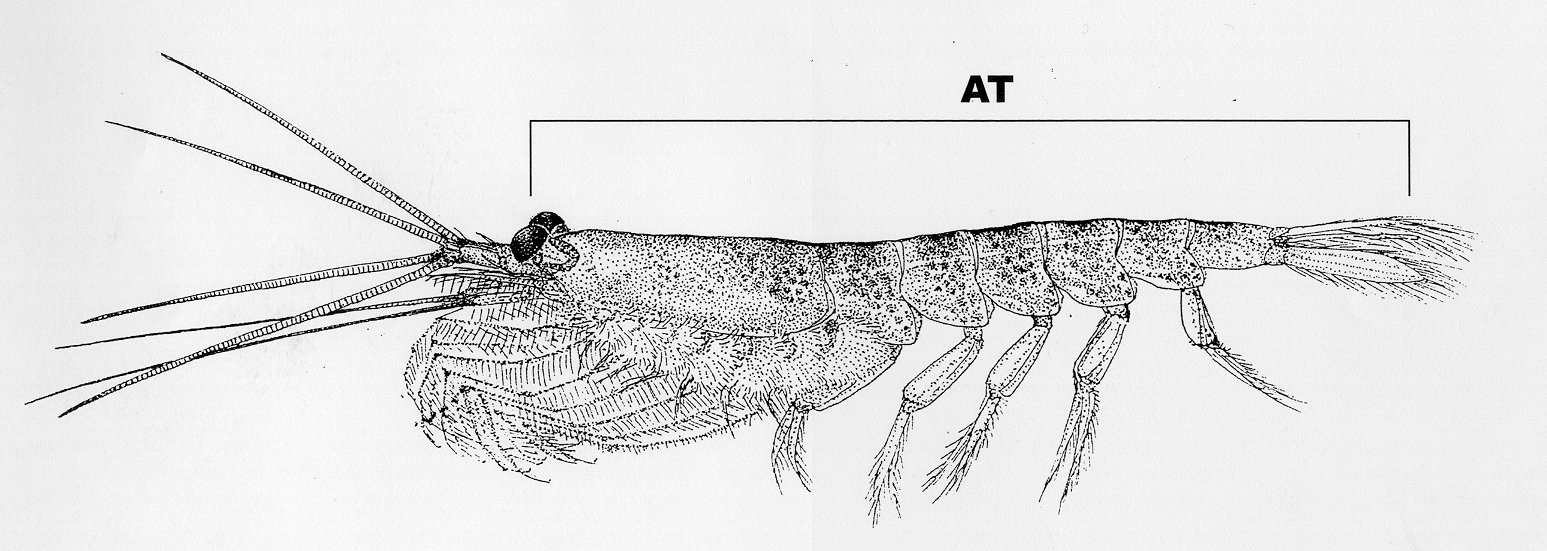 .
The standard unit is given in mm below , with an accuracy of 1 mm
size classes. All measurement on each vessel should be done by one
person to remove observer variation (see Watkins et al. 1986). Samples
which contain less than 150 krill are used in total for length measurements
and maturity stage identification. For larger krill catches a minimum of
150 krill shall be measured and staged.
.
The standard unit is given in mm below , with an accuracy of 1 mm
size classes. All measurement on each vessel should be done by one
person to remove observer variation (see Watkins et al. 1986). Samples
which contain less than 150 krill are used in total for length measurements
and maturity stage identification. For larger krill catches a minimum of
150 krill shall be measured and staged.
Maturity Stages
Krill sex and maturity stages should be identified using the classification
of Makarov and Denys (1981, BIOMASS Handbook)
Measurements of salps
If possible all salps should be removed from samples smaller than 1 litre
and counted. From larger samples a random subsample of 1 litre should be
taken (see above). Please note the different species Salpa thompsoni
and Ihlea racovitzai as well as the different forms (aggregate/solitary).
If possible a minimum of 100 specimens per species should be measured.
The internal body length (see figure and Foxton 1966)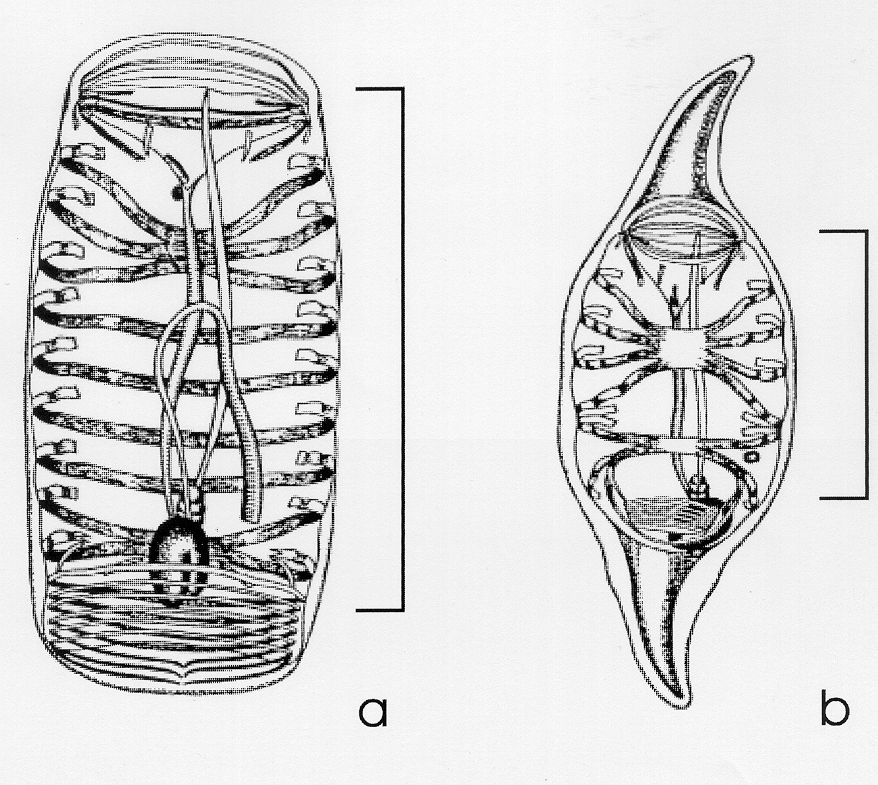 should be measured to the mm below with an accuracy of 1 mm size classes.
should be measured to the mm below with an accuracy of 1 mm size classes.
Other zooplankton from the RMT8 samples
If possible all other macro-zooplankton should be identified to the species
level, either from fresh material immediately after the catch or from preserved
samples. Special attention should be given to other euphausiid species
and early life history stages of fish species. After sorting the larger
organisms from the sample/subsample, the smaller constituents (e.g. krill
larvae) should be sorted using dissecting microscopes. In case of high
krill larvae abundance further splitting into subsamples might be necessary.
If possible the sample or subsample of other zooplankton should be preserved.
If possible length measurements should be carried out for key species
like Thysanoessa macrura (tip of the rostrum to tip of the telson,
mm below, 1 mm size class) and other euphausiid or fish species.
Preservation of krill
It is important that samples of krill are preserved for checking or future
studies. It is recommended that if sufficient krill are available then
the following strategy should be adopted.
-
a sample of 50 krill should be preserved in ethanol for genetic studies.
A minimum of 90% ethanol with a volume 10 times the volume of krill is
recommended
-
a subsample of krill which have not been processed should be preserved
in formalin as a back-up data set
-
the krill that have been measured and staged should be preserved in formalin.
Preservation of other zooplankton
A subsample of other zooplankton should also be preserved in formalin when
possible.
Data Entry
All relevant data should be entered onto computer prior to the termination
of the cruise. Electronic data sheets that look like this
will be provided.
This page is being developed by Jon Watkins
, Volker Siegel and So Kawaguchi
Page last updated on 17 November 1999



 .
The standard unit is given in mm below , with an accuracy of 1 mm
size classes. All measurement on each vessel should be done by one
person to remove observer variation (see Watkins et al. 1986). Samples
which contain less than 150 krill are used in total for length measurements
and maturity stage identification. For larger krill catches a minimum of
150 krill shall be measured and staged.
.
The standard unit is given in mm below , with an accuracy of 1 mm
size classes. All measurement on each vessel should be done by one
person to remove observer variation (see Watkins et al. 1986). Samples
which contain less than 150 krill are used in total for length measurements
and maturity stage identification. For larger krill catches a minimum of
150 krill shall be measured and staged.
 should be measured to the mm below with an accuracy of 1 mm size classes.
should be measured to the mm below with an accuracy of 1 mm size classes.